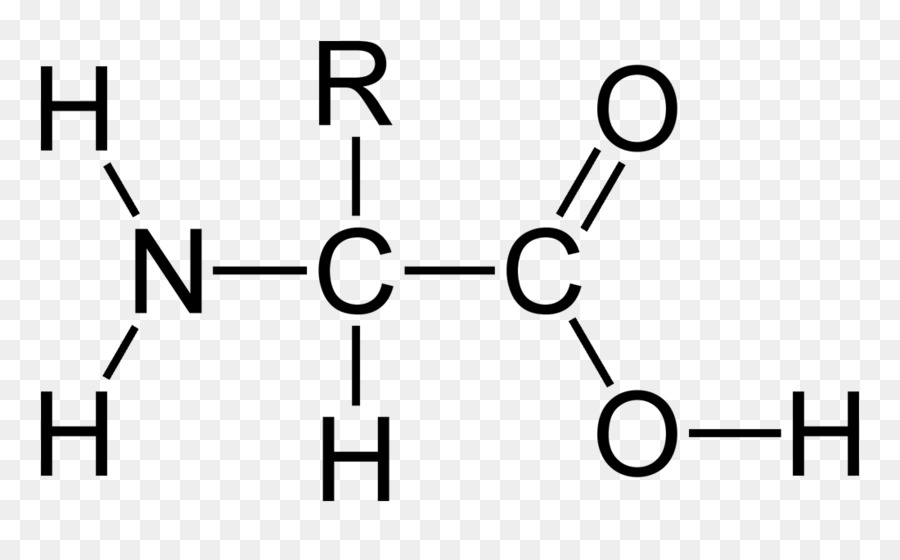
a) General structure of the amino acids. An amino group and carboxyl... | Download Scientific Diagram

Conformational Changes Induced by Methyl Side-Chains in Protonated Tripeptides Containing Glycine and Alanine Residues | The Journal of Physical Chemistry A
Chemistry Cartoon png download - 1100*662 - Free Transparent Glycine png Download. - CleanPNG / KissPNG

Glycine (Gly, G) Amino Acid Molecule. (Chemical Formula C2H5NO2) Stock Vector - Illustration of nitrogen, chemistry: 238491888

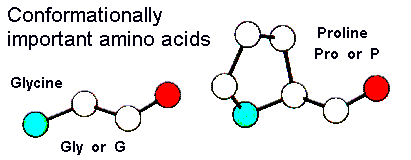
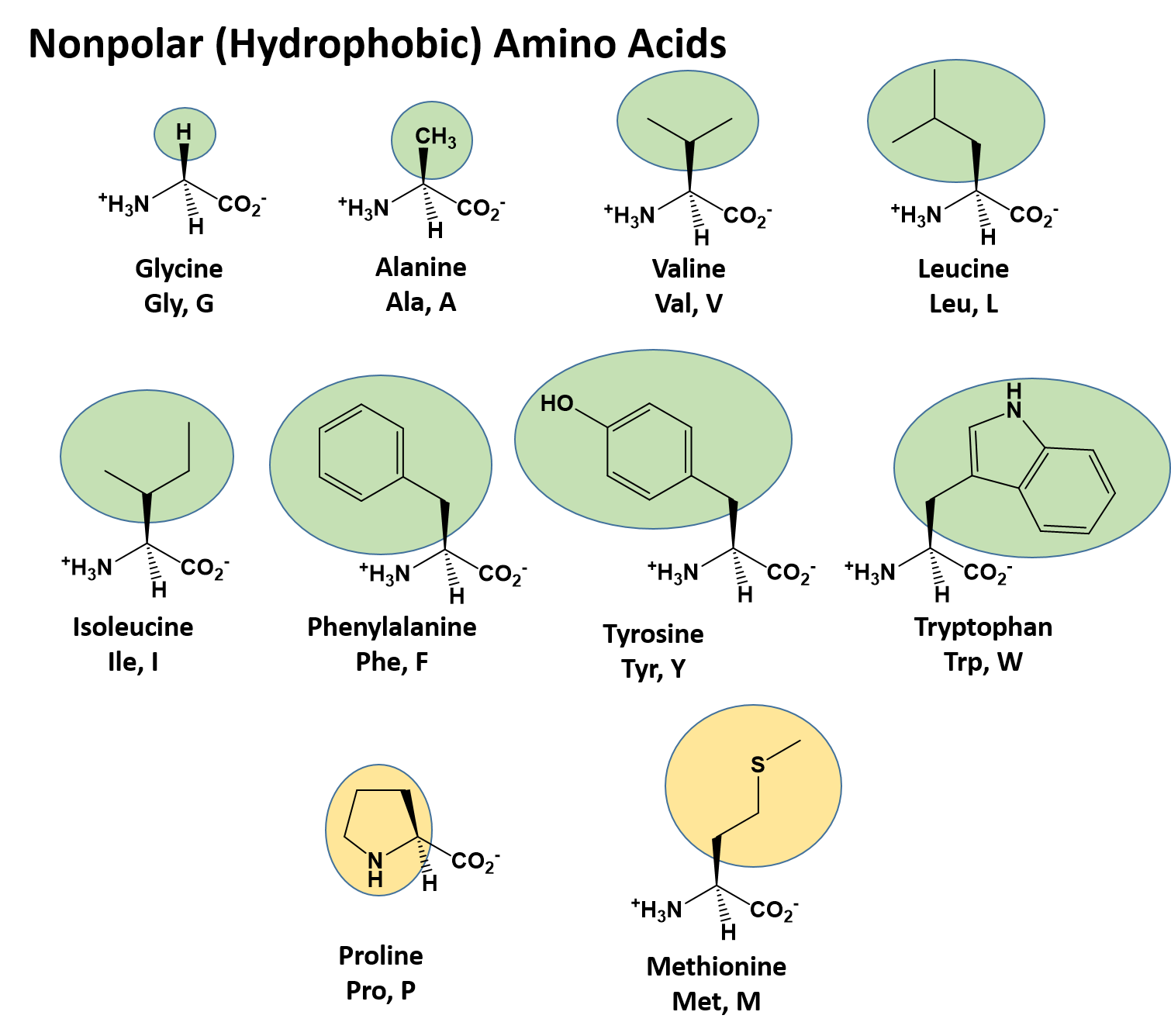
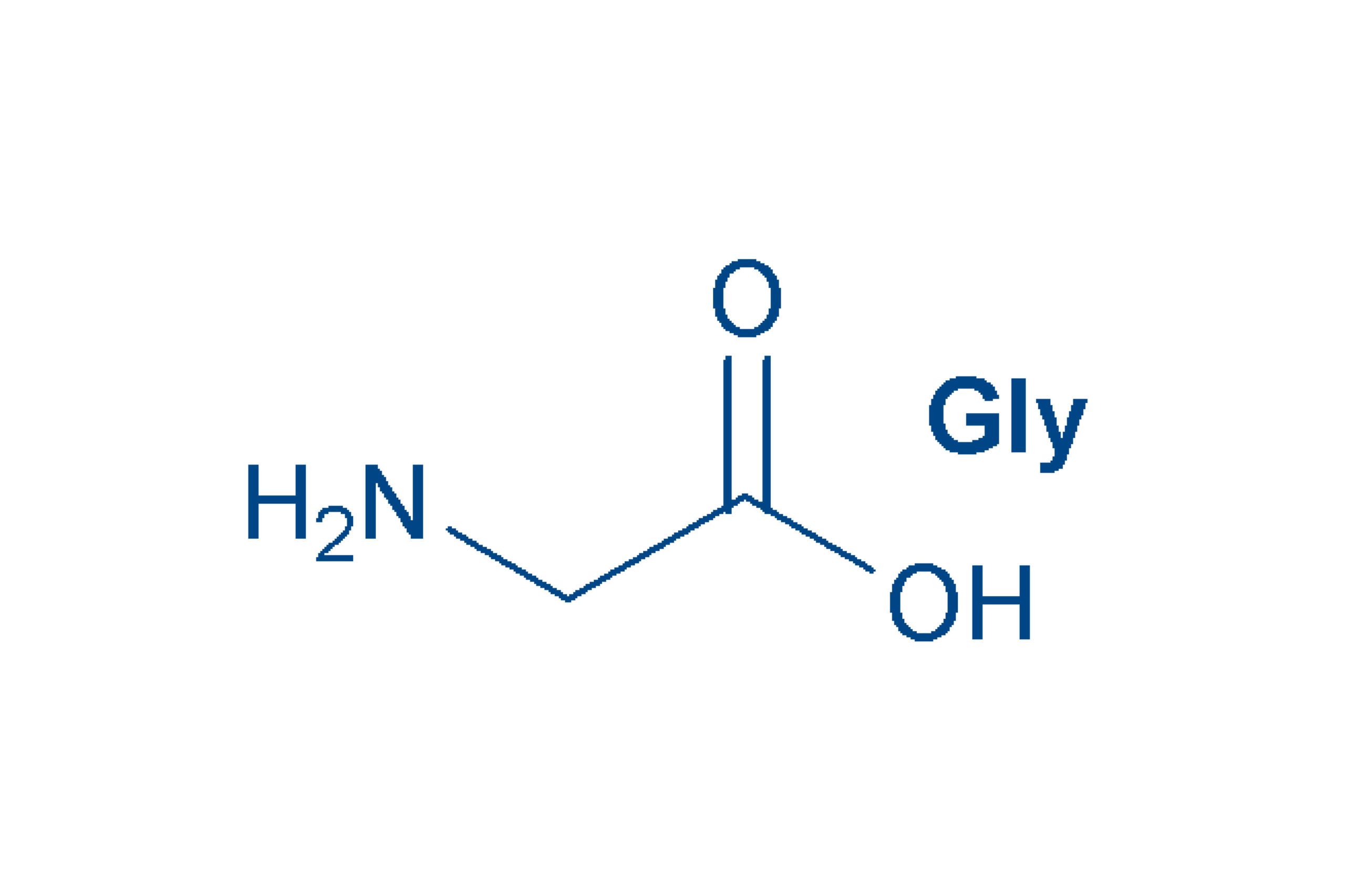
Glycine (abbreviated as Gly or G) is the amino acid that has a single hydrogen atom as its side chain. It is the simp… | Chemistry, Hydrogen atom, Organic chemistry

Glycine (Gly, G) Amino Acid Molecule. (Chemical Formula C2H5NO2) Is An Amino Acid That Has A Single Hydrogen Atom As Its Side Chain. It Is The Simplest Stable Amino Acid. Ball-and-stick Model,

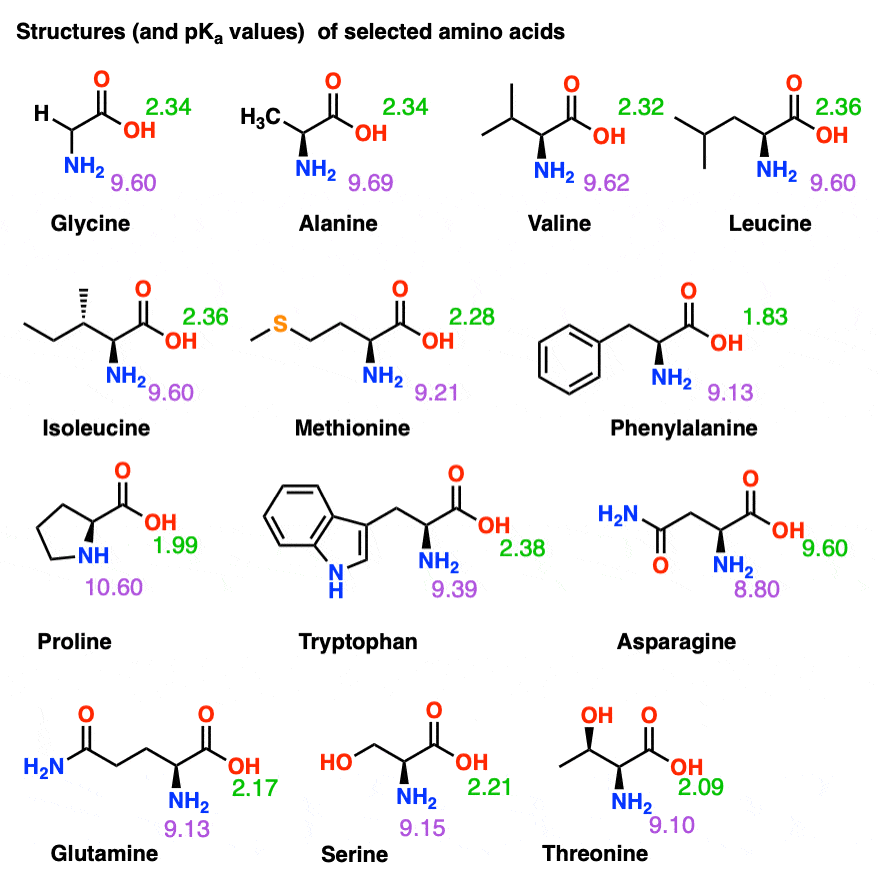
2. The structure of amino acid molecules. The simplest amino acid is... | Download Scientific Diagram