OneTouch Vibe Plus Insulin Pump Earns FDA Approval And Health Canada License And Is First Pump Integrated With The Dexcom G5 Mobile Continuous Glucose Monitor
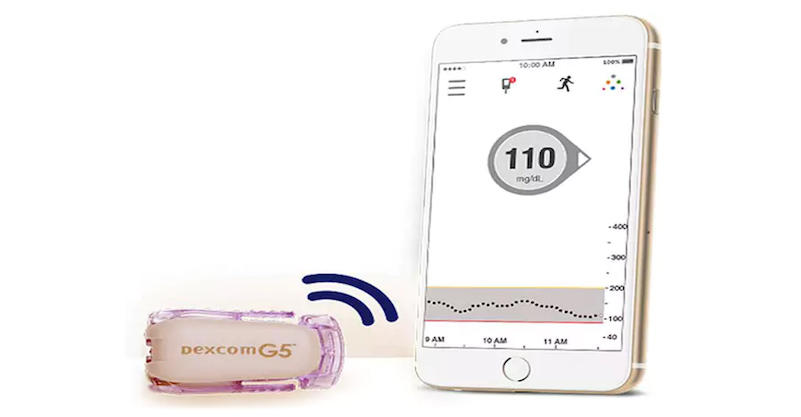
FDA approves diabetes device, Dexcom G5 Mobile CGM System, which aims to simplify blood sugar testing, dosing - CBS News
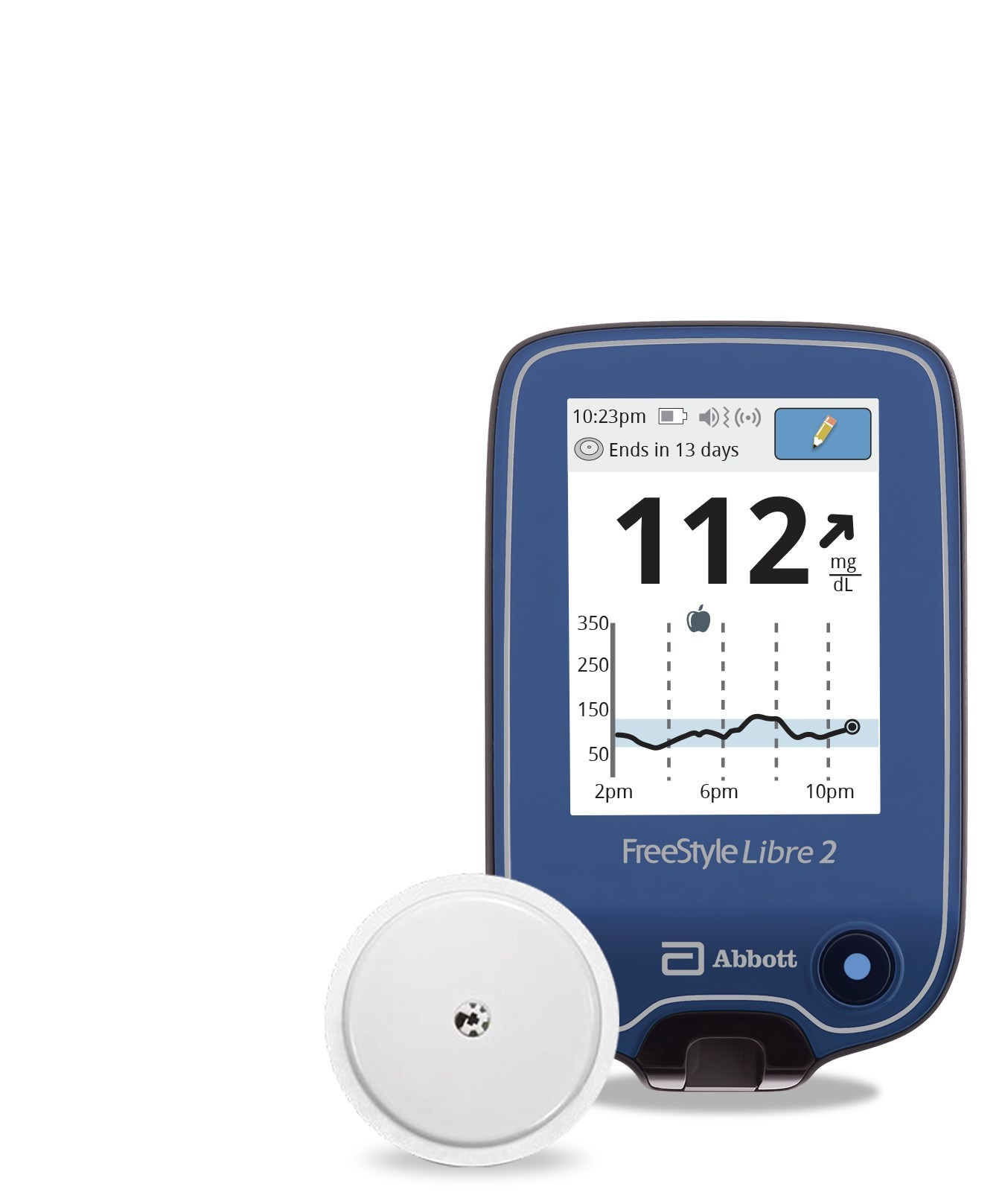
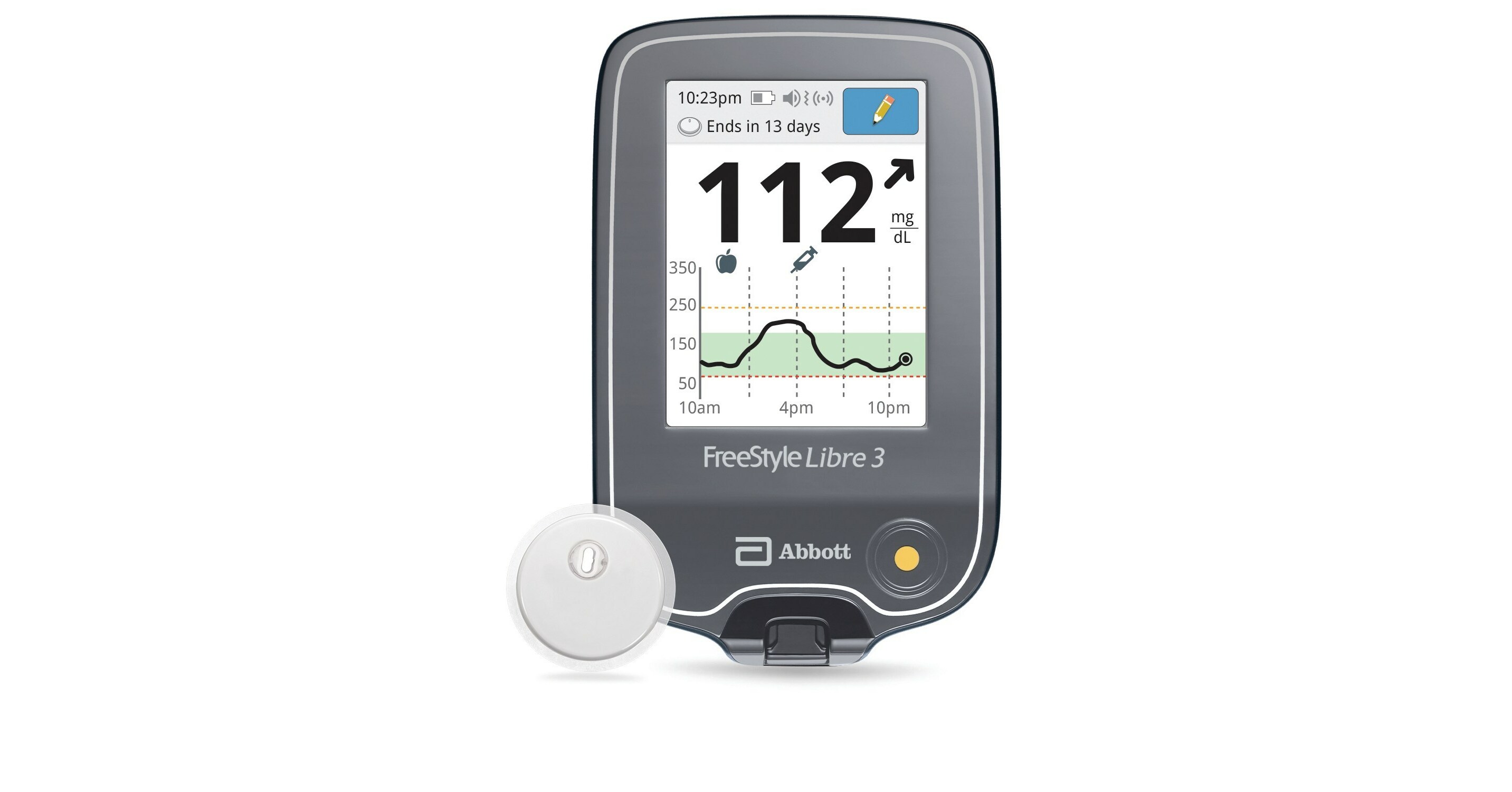
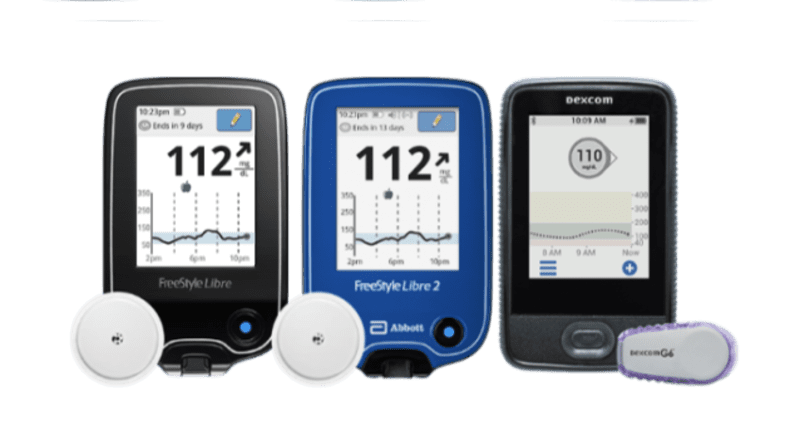
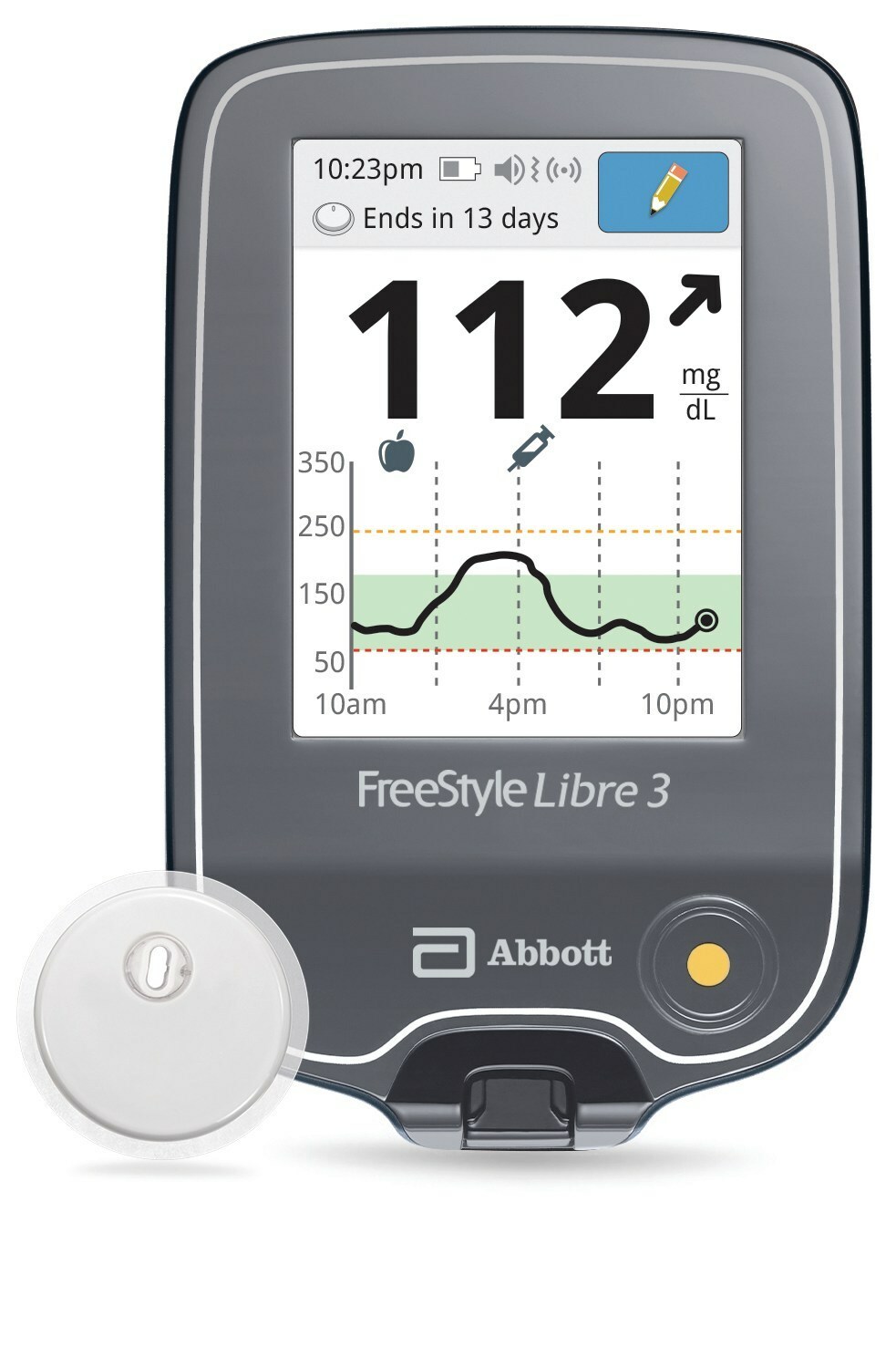
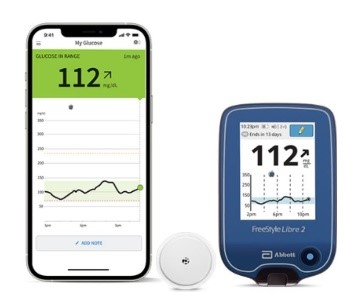
Abbott's FreeStyle® Libre 2 iCGM Cleared in U.S. for Adults and Children with Diabetes, Achieving Highest Level of Accuracy and Performance Standards - Jun 15, 2020

For People With Type 1 Diabetes, An 'Artificial Pancreas' Is Almost Here : Shots - Health News : NPR

Abbott's Freestyle Libre system becomes first CGM to be FDA cleared for use without fingersticks | MobiHealthNews



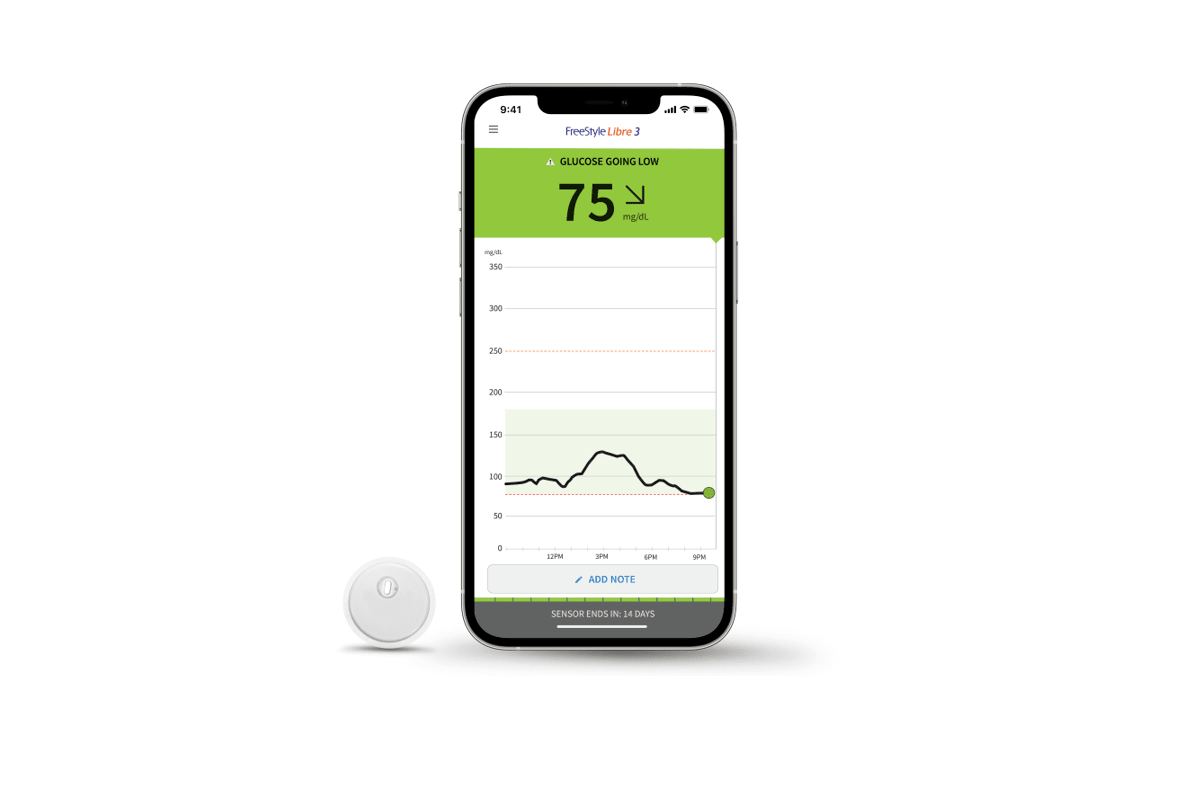








:max_bytes(150000):strip_icc()/Eversense-CGM-768x499-d920f1a66ddc42729d274ec509603bf6.jpeg)